Ovarian cancer 'game changer' drug
- by Alex Matthews-King, Health Correspondent
- •
- 01 Jun, 2018
which holds back disease's return gets first NHS fund approval
Thousands of women could benefit from a “game changing” ovarian cancer drug which can prevent the disease recurring for as much as a year and a half after it was approved for NHS use.
As many as 850 women a year could receive niraparib following its recommendation by the National Institute for Health and Care Excellence (Nice) for inclusion in the NHS Cancer Drugs Fund (CDF).
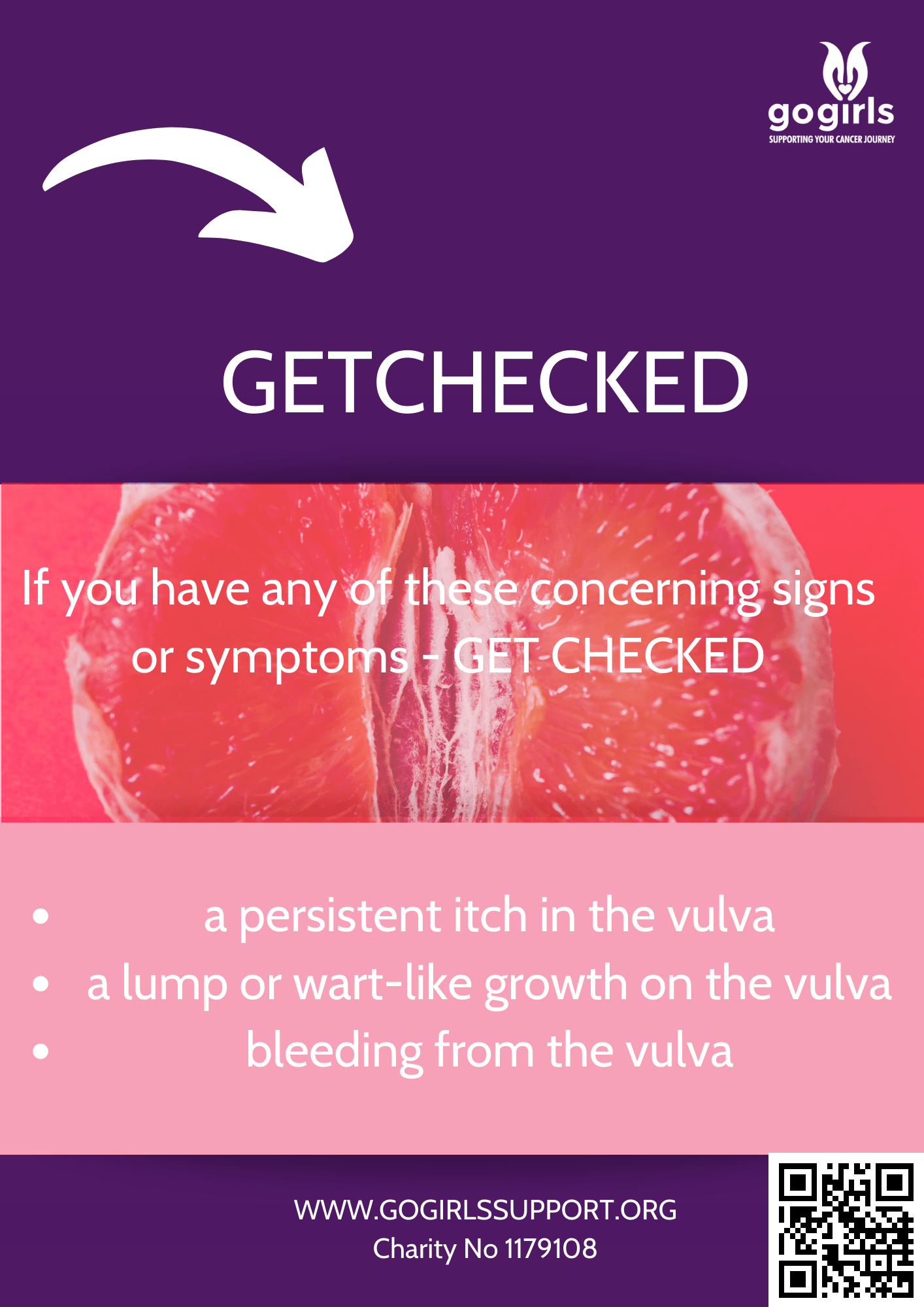
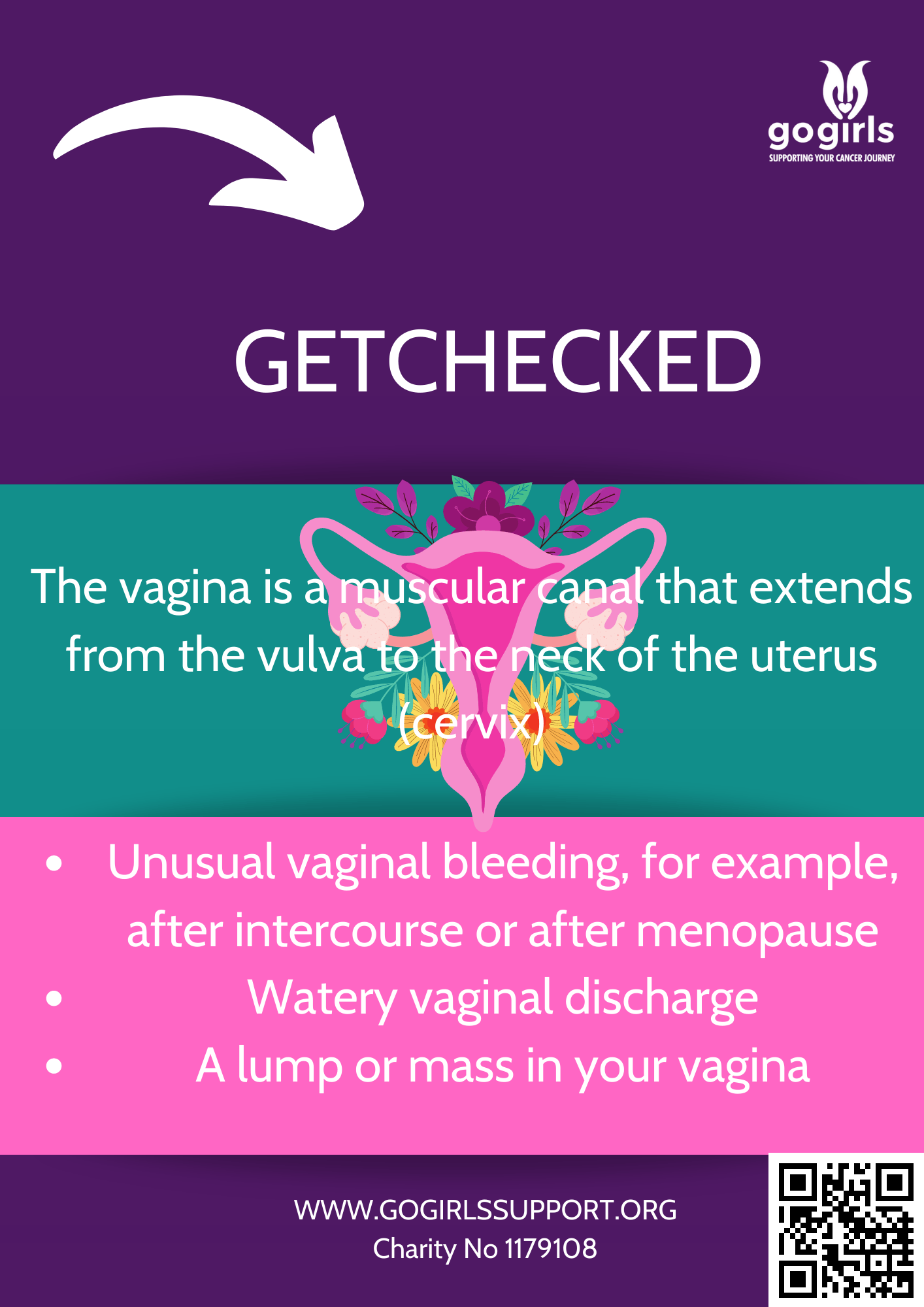
The targeted treatment is available to women with ovarian, fallopian tube or primary peritoneal cancers, which have recurred after two or more courses of chemotherapy.
Until now their treatment options have been highly limited and Nice says only a third of women survive five years after an ovarian cancer diagnosis.
CDF support will allow the NHS to collect detailed information on how the niraparib affects their cancer’s growth and long-term survival and assess its cost effectiveness.
Ovarian cancer is one of the most common types of cancer in women. In 2015, 6,198 people were diagnosed with the disease in England.
UK rates are among the highest in Europe but the country has one of the lowest survival rates.
Rebecca Rennison, director of public affairs and services at Target Ovarian Cancer, said: “Today’s announcement is a game changer in ovarian cancer.”
She added that the only new treatments in recent years have been restricted to women whose cancers meet very strict criteria, while niraparib could benefit many more women.
Jane Howarth, 54, from Manchester, has been taking niraparib for nine months.
She said: “When I was first diagnosed with ovarian cancer in 2015, my first words were, ‘But I’ve got children!’
“They were 11 and 14 at the time. I am now told that the cancer is advanced and incurable, but last September I started taking niraparib – and now I am at home, stable and well.
“I am there for my older daughter as she goes through her A-levels. I am there for my younger daughter as she navigates adolescence. We can make plans, and that is invaluable. I believe all women living with this disease should have that, too.”
Clinical trial results showed that niraparib delayed cancer growth by between six and 15.5 months more than a placebo, depending on a woman’s genetic profile.
However, the final results on overall survival were not available so it was not clear whether niraparib increased the length of time people live, meaning it could not receive definitive NHS approval.
The once-a-day pill works by inhibiting two proteins involved in DNA repair to prevent cancer growth.
Meindert Boysen, director of the centre for health technology evaluation at Nice, said: “The outcome for women with ovarian cancer is generally poor, with less than 35 per cent surviving for five years after diagnosis.
“We are pleased to see the inclusion of niraparib in the Cancer Drugs Fund as it will give women early access to this treatment while uncertainties in the clinical evidence can be addressed through the collection of additional data.”
Read full articleRecent Articles

In the process of becoming a lawyer, Jessica has played a pivotal role in assembling and guiding several businesses on a multitude of levels that assist in promoting ethical, social and industry standards. Alongside providing hands-on support to businesses, Jessica is a founder of charity Bona Fide, where she established and drove forward the ‘Beyond Bluewashing’ initiative, to bring public attention to the unethical corporate world and its effects on society. In addition, she has published many academic journals, articles and law reviews in the area of business law, as well as encouraging corporate philanthropic encouragement. Jessica is a keen advocator for causes close to her heart, one of which is related to GO Girls, as she is passionate about propelling forward awareness on these overshadowed cancers that impact many globally.